Newsletter June 2025
The nationwide virtual tumourbank project was launched 15 years ago by the Belgian Minister of Health, Ms. Laurette Onkelinx (initiative 27 of the Belgian Cancer Plan). The Belgian Virtual Tumourbank (BVT network) encompasses the tumour biobanks from eleven Belgian university hospitals (a list of these hospitals is available on the BVT website) that collect and store residual human tumour samples locally. In order to facilitate the search for tumour samples scattered among different Belgian institutions, data collected at sample level are made available for researchers via the online BVT catalogue (BVTc) application. A high quality of the data on the tumour samples requested by scientists for research in oncology is guaranteed by automatic and manual controls performed by the BVT project team at the Belgian Cancer Registry.
_____________________________________________________
Sample availability request
To check if specific samples for your research are available in our catalogue, you can complete the sample availability request form (Figure 1) on the BVT website. This document enables verification of the number of samples available in the BVT catalogue (BVTc), along with the contact details of the local biobanks hosting the samples of interest.

At this moment (June 2025), a total of more than 143,000 registrations including 125,910 primary tumour samples and 16,804 metastasis samples, are available in BVTc.
So far, we have received 51 Sample Availability Requests (SAR). Seven of the requests originated from abroad, including the United Kingdom, France and Portugal.

Figure 1: Preview of the online Sample Availability Request (SAR) form
_____________________________________________________
The requests in numbers
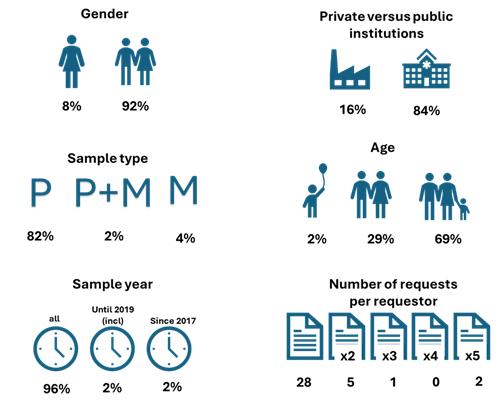
Only four (8%) requests explicitly asked for female samples while no gender
specification was made for the remaining requests. Eight (16%) requests came from industry
, while the other requests were from public institutions. For 49 requests (96%), no sample year was specified. Fifteen (29%) requests were for samples from adult patients, including 2 with additional age specifications (> 50 years and 40-48 years). Samples from children and adolescents were asked only in one request (2%) and for 35 requests (69%) there was no restriction on age.
The majority of the requests concern primary tumour samples (82%). For two requests (4%) only metastasis samples were needed and one request asked for both primary tumour and metastasis samples. For the remaining six requests (12%) no clear specification of the sample type could be made based on the submitted form.
Most requestors (n=28) completed the form only once. However, two requestors submitted the form five times, one requestor three times and five requestors twice.
_____________________________________________________Which sample sites and conservation modes are requested?
Most requested tumour sample localisations were lung, liver, colorectal and breast. For 11 requests (22%) multiple sample localisations were needed while for 13 requests (25%) no specification of the localisation was provided.
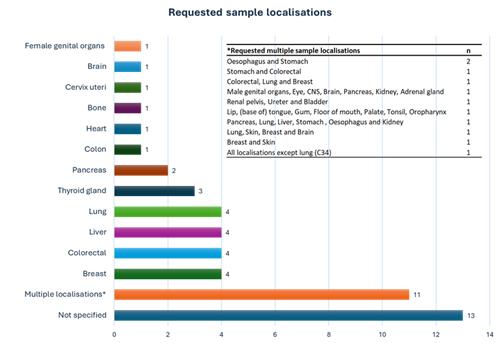
Figure 2: Requested sample localisations
Inset: Specification of the requests with multiple sample localisations
The most frequently requested tumour samples were samples embedded in paraffin (21%), followed by frozen samples (14%) and both frozen samples and paraffin samples (14%). For 35% of the requests no specification of the conservation mode was made (figure 3).
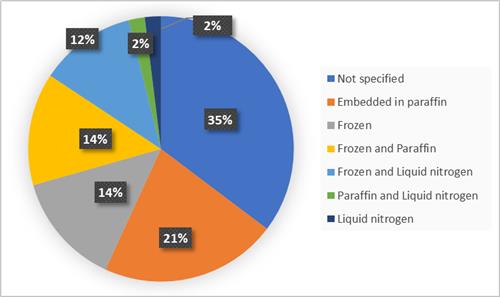
Figure 3: Requested modes of conservation
_____________________________________________________
How about exclusion criteria?
In 37 requests (73%) no specific exclusion criteria were mentioned. An overview of the exclusion criteria in the remaining requests is presented in the table below.
Exclusion criteria | n | % |
Auto-immune disease, immunosuppressive drugs | 5 | 10 |
Samples obtained before 2017 | 1 | 2 |
Samples obtained after 2020 and no clinico-pathological data available | 1 | 2 |
Only patient matched pairs (brain metastasis with primary lung, skin or breast tumour) | 1 | 2 |
Active infection, liver, lung of kidney disease, active neoplasm diagnosed during past 5 years | 1 | 2 |
Inflammatory disease linked to the bowel (IBD, Crohn,...) and also auto-immune | 1 | 2 |
Viral hepatitis | 1 | 2 |
Lung cancer | 1 | 2 |
Prior history of cancer, treatment prior to sample collection, hepatitis B, hepatitis C or HIV infection at time of sampling | 1 | 2 |
Tumours not related to Von-Hippel Lindau disease. | 1 | 2 |
Table 1: Overview of the exclusion criteria
_____________________________________________________


Scan the QR-code and follow us on LinkedIn for regular updates and news about the BVT.
_____________________________________________________