FAQ
What do I have to register?
Disease-free interval / progressive disease / relapse
Basis of diagnosis when coding the histology of regional lymph node metastases
Primary tumour localisation: do lymphomas always have the topography code C77.x?
Synonyms for an (invasive) spinocellular epithelioma
Why can't I enter a pM0?
Malignant melanomas: do I enter the Clark or the Breslow?
Can a Transurethral resection (TUR) of the bladder treatment be coded using code 10? Can a pTNM classification be determined based on a TUR of the bladder?
Can a Transurethral resection of the prostate (TURP) treatment be coded using code 10? Can a pTNM classification be made based on a TURP?
How do you handle the TNM changes with respect to the differentiation grade of prostate tumours?
Why is there no pT1 for prostate tumours?
How do you code neuroendocrine tumours?
How do you code Gastrointestinal Stromal Tumours (GISTs)?
What about dysplasia?
How can I register foreigners via WBCR?
How do you code papillary tumours correctly?
How do you communicate WBCR registration corrections (this guideline applies to all WBCR users as from the operational year 2013)?
How do you communicate confidential, personal details?
What are the consequences of the new classification for lung tumours (IASLC-2011) when registering lung tumours?
Do moles (hydatidiform moles, invasive moles, etc.) need to be registered?
1. What do I have to register?
In the context of compulsory statutory cancer registration in Belgium, the following diagnoses must be registered on the cancer registration forms provided for this purpose for new diagnoses (established by the Belgian Cancer Registry based on international guidelines):
- all new diagnoses of cancer, in other words, all malignant tumours, invasive or in situ
- all haematological tumours, including myeloproliferative diseases, myelodysplastic syndromes
- all tumours of the central nervous system irrespective of tumour behaviour (benign, low malignant potential, malignant)
- all urothelial cell tumours (low malignant potential, in situ and invasive)
- ovary: all malignant and borderline malignant epithelial tumours
Exceptions:
- basal cell carcinomas do not need to be registered (the basal cell carcinomas delivered by anatomical pathologists are sufficient for the cancer registry; but this is the only tumour to which this applies); spinocellular tumours of the skin must therefore be registered!
- haemangiomas of the central nervous system do not have to be registered.
If two or more tumours are diagnosed (e.g. in different organs, bilateral breast tumours etc.) a separate form must be completed for each tumour.
Top
2. Disease-free interval / progressive disease / relapse
The following definitions are used to register follow-up data on the cancer registration forms provided for this purpose:
Disease-free interval: clinically no more cancer can be found. One often also refers to 'total or complete remission'. Important is whether doctors consider the patient to be tumour-free or not.
If the same cancer is diagnosed following a disease-free interval, we call it a relapse (local or distant; for a distant relapse, the term metastasis is used).
A progressive disease implies a further negative evolution of the disease without a disease-free interval. A progressive disease can occur e.g. following an initial stable situation or a partial remission.
For the sake of completeness we also include partial remission here. This means that the patient has responded well to the established treatment with regression of the tumour, but is not completely tumour-free.
Top
3. Basis of diagnosis when coding the histology of regional lymph node metastases
Regional lymph node metastases are registration-technically not considered as distant metastases for the determination of TNM stage. In the TNM classification you will find them under N for Node. Since they are actually considered as local metastases, you may use '3' for basis of diagnosis (histology metastasis).
Please note: never enter C77.x as a topography code when coding regional lymph node metastases. You must use the code for the organ in which the primary tumour is located. If it is not known from which primary tumour the lymph node metastases originate, use code C80.9 ('unspecified localisation / primary localisation unknown').
Top
4. Primary tumour localisation: do lymphomas always have the topography code C77.xl?
Lymphomas are cancers of the lymphatic tissues. The lymph nodes (C77.X) are usually coded as the primary tumour localisation (nodal localised lymphomas). However, lymph node cells naturally circulate in the blood and the lymph. This is how these cells move to other lymph nodes. Lymphomas spread in the same way. This is why lymphomas are found in different parts of the body, both in and outside the lymphoid tissue and explains why a lymphoma can also primarily originate in an organ. In that case, the organ is coded as the primary tumour localisation (extranodal localised lymphomas). Examples of extranodal localised lymphomas are lymphomas that are first diagnosed in the stomach, tonsils, skin etc.
Lymphomas do not originate in the bone marrow (C42.1). The discovery of a lymphoma in the bone marrow indicates an advanced stage of the disease which could be confirmed by the associated Ann Arbor stage. Tip: when bone marrow defects are found in a patient with a lymphoma, it should be verified whether it concerns a lymphoma that was discovered in an advanced stage. It could also be a lymphoma that is already known for a while (with an incidence date that could be months to years before the positive bone marrow sample) in which the evolution to bone marrow invasion has occurred.
If you do not know where the disease originated (organ or lymph nodes), use the code C77.9.
Top
5. Synonyms for (invasive) spinocellular epithelioma
Few tumours are assigned so many names. It concerns a spinocellular epithelioma if you come across one of the following terms:
- spinocellular carcinoma
- spinocellular epithelioma
- spino
- epi-epi
- epidermoid epithelioma
- epidermoid carcinoma
- squamous cell carcinoma
- squamous cell epithelioma
- the code 8070 (/3)
Top
6. Why can't I enter a pM0?
Technically a pM0 is almost impossible. This would imply that one has cut the whole bodyup, examined it under the microscope and has not found a tumour anywhere. From a scientific point of view that would be extremely interesting but in reality it is not done, except in the case of an autopsy. Therefore a pM0 is only possible if an autopsy has confirmed the absence of metastases and one has entered '1' as the basis of diagnosis.
Unless one possesses histological evidence of metastases, one records (if no autopsy was carried out) the pM status as follows:
- For tumours before 2010: enter pMx (up to and including TNM 6th edition)
- For tumours as of 2010 (as of TNM 7th edition): leave the pM field empty
It is best to enter cM0 so as not to lose the information that no distant metastasis was found.
Top
7. Malignant melanomas: do I enter the Clark or the Breslow?
The TNM classification is better than the Clark or the Breslow! It provides much more relevant information. If you still want to enter the Clark or the Breslow it is best to use the Breslow classification because it provides more useful information than the Clark classification.
The Breslow classification that you may find in certain documents can help you to establish a TNM because it provides some of the information required to do so.
Top
8. Can a Transurethral resection (TUR) of the bladder treatment be coded using code 10?
Can a pTNM classification be determined based on a TUR of the bladder?
A TUR (transurethral resection) of the bladder can be an effective method for fully (in one piece) removing a (small) bladder polyp. These polyps are the most frequent types of bladder tumours. The pathologist can often use the TUR to effectively establish a pTNM. The TUR of the bladder can then be considered as a real treatment for bladder polyps and is coded accordingly if the bladder tumour is fully treated in this way.
If it appears impossible to treat the bladder tumour with a TUR, and if a (partial) cystectomy follows, we propose that the TURB be coded as '80' (other) and the cystectomy as '10' (surgery) (in line with point 9).
Top
9. Can a Transurethral resection of the prostate (TURP) treatment be coded using code 10? Can a pTNM classification be made based on a TURP?
During a TUR of the prostate, prostate tissue in the prostate capsule is peeled away sliver by sliver. The prostate capsule is left in place and a considerable quantity of prostate tissue also usually remains behind. The objective of a TURP is usually to help a urinary problem resulting from the urethra being pressed by an (age-related) increase in prostatic glandular tissue.
It is impossible for the pathologist to establish a pTNM using the prostate slivers obtained. He or she cannot make an assessment about invasion of the prostate capsule, invasion of adjacent organs etc.
As a rule a TURP is not considered as a curative treatment for prostate cancer. Prostate cancer can be treated with a radical prostatectomy or radiotherapy. Therefore only a radical prostatectomy is coded using code 10.
A TURP is coded as '80: other' and in the corresponding field one enters 'TURP'.
N.B.: for older patients a TURP can sometimes be proposed as palliative treatment (only to solve the urinary problem, not to treat the carcinoma itself).
N.B.: Sometimes during a TURP treatment for a urinary problem caused by prostate hypertrophy, a prostate carcinoma is discovered totally unexpectedly.
Top
10. How do you handle the TNM changes with respect to the differentiation grade of prostate tumours?
In the 2nd print of their 7th edition, the TNM's authors have unexpectedly changed the differentiation grade, based on the Gleason score.
G Histopathological Grading according to the TNM 7th edition (2nd print).
G1 Well differentiated (slight anaplasia) (Gleason 2 - 6)
G2 Moderately differentiated (moderate anaplasia) (Gleason 7)
G3-4 Poorly differentiated/undifferentiated (marked anaplasia) (Gleason 8 - 10)
This leads to data that is incorrect or difficult to compare in databases.
Therefore we suggest entering the Gleason grade from now on as well as the differentiation grade. In the WBCR this could be entered in the 'Other classification' field, 'Type: Other' and in 'Stage', write in full Gleason number= number + number (e.g. Gleason 7=3+4).
Sources that supply in batch (extraction from own database) can also enter this (preferably in full) in the column 'Other classification + stage'.
Top
11. Why is there no pT1 for prostate tumours?
For all organs the pTNM can only be established following the resection of the primary tumour. A biopsy that allows the highest pT and/or pN category to be established can also result in a correct pTNM classification.
However, a prostate biopsy never allows the highest pT category to be established given that no information about the extent of the primary tumour, possible invasion of the capsule and/or other organs can be obtained from a puncture.
Therefore a pT for a prostate carcinoma can only be entered following a total radical prostatectomy (traditional or laparoscopic, whether or not robot-assisted). It then always concerns a tumour that will be classified at least as a pT2 (and possibly pT3, pT4).
Confusion arises because when determining the cT1 in prostate tumours the results of microscopic examinations is used (a cT1a and a cT1b are based on the examination of TUR-PROSTATE slivers; a cT1c is based on the examination of needle biopsies).
Important: a cT1 may only be entered if the tumour is not palpable or visible on imaging. If it is palpable or visible on imaging, it concerns at least a cT2 tumour!
Top
12. How do you code neuroendocrine tumours?
Is there a difference between coding a neuroendocrine tumour and a neuroendocrine carcinoma?
There is increasing support for considering ALL neuroendocrine tumours as malignant tumours. Most (all?) of them present the ability of metastasising. Some neuroendocrine tumours are only labelled as malignant when metastasis occurs... This is inconsistent with guidelines that apply to other tumours.
Apparently the WHO is following this increasing support, because in its latest update of the ICD-O 3 (Nov 2011) the term neuroendocrine tumour, grade 1 and 2, was added to the list of tumours with behaviour /3.
'Grade 1' and 'grade 2' may be explicitly mentioned here, and we know from experience that a number of pathologists fail to mention the grade in their protocol. However, on the basis of this WHO update we believe there is sufficient argument for reading any 'neuroendocrine tumour' (in other words a neuroendocrine tumour NOS) as 'neuroendocrine carcinoma' and therefore allocating behaviour /3.
How is a pheochromocytoma/paraganglioma coded?
A pheochromocytoma is a neuroendocrine tumour that originates from the chromaffin cells and is located in the adrenal glands in most patients. In approximately 10% of patients the pheochromocytoma is situated outside the adrenal glands in a paraganglion; this is referred to as a paraganglioma.
As already mentioned above, there is increasing support for considering ALL neuroendocrine tumours as malignant tumours. Most (all?) present the ability of metastasising. Pheochromocytomas are often considered as benign until a metastasis is suddenly discovered...This is inconsistent with the guidelines that apply to other tumours.
We also suggest assigning behaviour /3 to all pheochromocytomas.
The problem remains of course, when the pathologist/clinician explicitly notes that it concerns a benign pheochromocytoma. One solution involves actually registering and coding these tumours, but with behaviour /0. In this way the tumour will be included in our database, which can be useful if metastases of this tumour initially considered as benign are detected later and the pheochromocytoma thus undergoes a 'behavioural change' over time.
The same reasoning can be applied to paragangliomas, which belong to the same group of tumours as pheochromocytomas.
Top
13. How do you code GastroIntestinal Stromal Tumours (GISTs)?
A TNM classification for GIST tumours was introduced in the 7th edition of the TNM. Like for neuroendocrine tumours, an increasing number of people are convinced to consider all GIST tumours as malignant (behaviour /3). Even the smallest GIST tumours appear to develop the ability to metastasise.
The TNM 4th edition supplement (belonging to the 7th edition TNM) also recommends that ALL GISTs be classified according to TNM even if they might be benign, because their behaviour is actually unpredictable.
The combination of the TNM, the organ in which the GIST tumour is localised and the mitotic index allows these tumours to be classified more effectively according to their prognosis.
CONCLUSION: all GIST tumours must be coded with /3 as of the incidence year 2010.
Top
14. What about dysplasia?
The term dysplasia presents many problems. Especially the terms serious/strong dysplasia, severe dysplasia and high-grade dysplasia in particular cause confusion. Moreover there are no objective criteria for distinguishing between severe/high grade dysplasia and a carcinoma in situ. It remains a fairly subjective interpretation by the pathologist. In any case the treating physician must be alert when diagnosing severe/high-grade dysplasia as well as when diagnosing carcinoma in situ given the difference between them is very minimal, even non-existent.
Therefore the Belgian Cancer Registry applies the following rule as of the incidence year 2010:
Severe dysplasia High-grade dysplasia Carcinoma in situ High-grade intra-epithelial neoplasia | | use behaviour /2 |
Please note: an intramucosal carcinoma is not a synonym for a carcinoma in situ. For oesophageal tumours an intramucosal carcinoma is always considered as T1 (in other words as an invasive tumour) whereas for colorectal tumours an intramucosal carcinoma is considered as a Tis (in other words a non-invasive tumour).
Top
15. How can I register foreigners via WBCR?
Strictly speaking, only those people domiciled in Belgium must be registered in the Cancer Registry since only those with their official residence in Belgium are included in the Belgian Cancer Registry statistics. However, we encourage oncological care programmes to register all cancer diagnoses (cf. recognition norms for oncological care programmes), regardless of the patient's place of residence.
Foreigners that are domiciled in Belgium:
These persons are included in Belgian incidence numbers and consequently must be registered. EU citizens are not obliged to apply for a National Register number but will often receive one when they register at the municipality. You can use this National Register number for the registration. If this patient does not have a National Register number, he or she can be registered using the health insurance number or own unique identification number. Belgium must be selected as the country code (code of the country in which the patient resides). If a 'warning' appears, you must note in the comments field that it concerns a foreigner with a place of residence in Belgium.
Foreigners that are not domiciled in Belgium:
Strictly speaking these persons do not have to be registered, as they are not included in our statistics. However we highly recommend the registration of these cases -in the care programme. This data may also be interesting for us if such patients later become domiciled in Belgium.
They can be registered with the (temporarily assigned) health insurance number or a unique identification number. As a country code you must select the country in which the patient is actually domiciled.
Top
16. How do you code papillary tumours correctly?
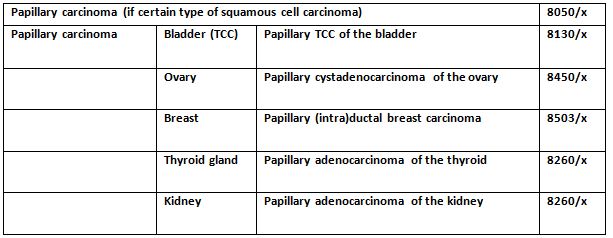
The above table can help identify the correct code for the correct tumour.
Top
17. How do you communicate WBCR registration corrections (this guideline applies to all WBCR users as from the operational year 2013)?
If you want to make adjustments to WBCR registrations that have already been submitted, you should enter the registration again via WBCR explicitly mentioning 'Corrected version' in the comments field. You could add which variable has been corrected (e.g. corrected version concerning cTNM and treatment). This means that the corrected information will immediately be integrated in our application.
However, if you have entered a registration under an incorrect SSIN (social security identification number) number, we ask you to first contact your Cancer Registry contact person by telephone so that the incorrect registration is immediately and fully deleted from our database. You can then re-enter the registration using the correct SSIN number.
Top
18. How do you communicate confidential, personal details?
The National Health Act of 13/12/2006 obliges the Cancer Registry to take strict organisational and technical security measures to guarantee the protection of data. For this reason we strongly urge you to NEVER SEND any non-anonymous patient data BY EMAIL given that this transfer method offers insufficient guarantee of privacy protection.
Just three methods are deemed secure:
Providing information to your Cancer Registry contact person by telephone: of course, this is only possible for a limited amount of information
Using a CD-ROM secured with a password and sent by registered post, to the attention of Dr. Liesbet Van Eycken, arts toezichthouder (supervisory physician) – Belgian Cancer Registry – Koningsstraat/Rue Royale 215 box 7 – 1210 BRUSSELS. The password must be communicated to your Cancer Registry contact person by telephone.
Via sFTP (secure File Transfer Protocol) whereby information exchanges between external parties and the Cancer Registry can be conducted securely. The user manual for this transfer method can be found among the downloads (manuals for SKR applications). A login and password (valid for just 14 days) can be obtained from your Cancer Registry contact person by telephone.
Top
19. What are the consequences of the new classification for lung tumours (IASLC-2011) when registering lung tumours?
In 2011, the International Association for the Study of Lung Cancer (IASLC) proposed a new classification for lung tumours (Journal of Thoracic Oncology – Volume 6, Number 2, February 2011). One of the main consequences for registering lung tumours is the recommendation that the term 'Bronchioloalveolar carcinoma' or 'BAC' no longer be used.
There is new terminology (ICD-O-3 codes remain unchanged):
- Adenocarcinoma, NOS : 8140/3
- Lepidic (predominant) adenocarcinoma, NOS (formerly BAC, NOS) : 8250/3
- Lepidic non-mucinous adenocarcinoma (formerly non-mucinous BAC) : 8252/3
- (Lepidic) mucinous adenocarcinoma (formerly mucinous BAC) : 8253/3
- Mixed mucinous and non-mucinous adenocarcinoma (formerly mixed mucinous and non-mucinous BAC) : 8254/3
- Adenocarcinoma in situ (formerly BAC in situ): 8250/2
Non-mucinous adenocarcinoma in situ: 8252/2
Mucinous adenocarcinoma in situ: 8253/2
Top
20. Do moles (hydatidiform mole, invasive mole, etc.) need to be registered?
A mole is a benign trophoblastic disease and is not included in cancerregistration.
However, apart from moles there are other trophoblastic tumours, several of which are malignant. The following trophoblastic diseases are subject to statutory compulsory Cancer Registration (and are also eligible for a Multidisciplinary Consultation):
Choriocarcinoma (9100/3)
Choriocarcinoma combined with other germ cell elements (9101/3)
Malignant trophloblastic teratoma (9102/3)
Epithelioid trophoblastic tumour (9105/3)
In addition to the Belgian Cancer Registry there is also a Belgian Registry for Trophoblastic Diseases. This registry collects - separately from the Cancer Registry - information about both benign (such as moles) and malignant trophoblastic diseases (such as choriocarcinomas). Participation in this trophoblast registry is not compulsory and the patient's permission must be obtained. The information registered can be found on the registry's website (http://www.mole-chorio-bgog.eu/fr). The registration of these diseases is encouraged in order to improve the information for patients with these rare diseases and to help doctors optimising their diagnoses, treatment and monitoring.
Registration of a malignant trophoblastic disease in the Cancer Registry is compulsory and it can also be registered in the Belgian Registry for Trophoblastic diseases. To date there is no information exchange between the two registries.
Top